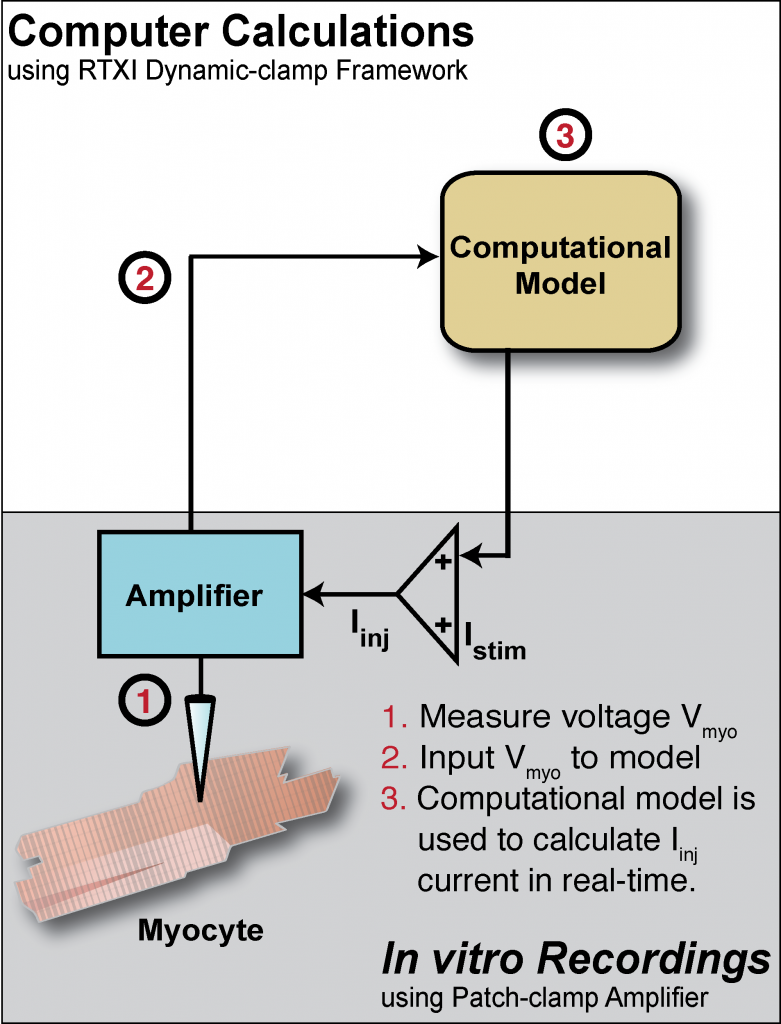
 Our experiments employ manual and multi-cell automated electrophysiological modalities, using induced pluripotent stem-cell derived cardiomyocytes (iPSC-CMs), often via real-time feedback between the experiment and the model (“dynamic clamp”). Dynamic clamp is a hybrid computational modeling and patch-clamp approach used in cardiac electrophysiology research. Custom software modules developed in the Real-Time eXperimental Interface (RTXI) framework (www.rtxi.org) are used to perform real-time computations and feedback to the patch-clamp amplifier.
Our experiments employ manual and multi-cell automated electrophysiological modalities, using induced pluripotent stem-cell derived cardiomyocytes (iPSC-CMs), often via real-time feedback between the experiment and the model (“dynamic clamp”). Dynamic clamp is a hybrid computational modeling and patch-clamp approach used in cardiac electrophysiology research. Custom software modules developed in the Real-Time eXperimental Interface (RTXI) framework (www.rtxi.org) are used to perform real-time computations and feedback to the patch-clamp amplifier.
Our laboratory performs a variety of dynamic-clamp experiments including:
Gap junction coupling of a computational model to an in vitro cardiac myocyte
To couple an in vitro cardiomyocyte with a computational model of a cardiac cell, membrane potential is recorded from the patch-clamped cell, while the membrane potential of the model is computed in real-time. A gap junctional current is then calculated and injected into the in vitro cardiomyocyte via the amplifier. The end result is real-time electrical interactions between the two ”cells” as if an intercellular gap junction physically coupled them.
Mimic pharmacological block by the scaling of membrane currents of an in vitro cardiac myocyte
A computational model, which describes the time and voltage dependent membrane current of a cardiac cell is used to calculate and inject a current to which will scale the desired membrane current in the in vitro cardiac myocyte. The end result is a real-time mimicking of pharmacological manipulations in the context of a whole cell.
- “Anthropomorphizing the Mouse Cardiac Action Potential via a Novel Dynamic Clamp Method.” Biophysical Journal. 97.10: 2684-2692. 2009
- “Rapid Genetic Algorithm Optimization of a Mouse Computational Model: Benefits for Anthropomorphization of Neonatal Mouse Cardiomyocytes.” Frontiers in Physiology. 3: 421. 2012.